The EU's Medical Device Regulation (EU) 2017/745 – Are You Ready for Huge Sweeping Changes? - In Compliance Magazine

Medical Devices Clinical Evaluation - Summary of Safety and Clinical Performance (SSCP) - Regulation (EU) 2017/745 - GMED Medical Device Certification

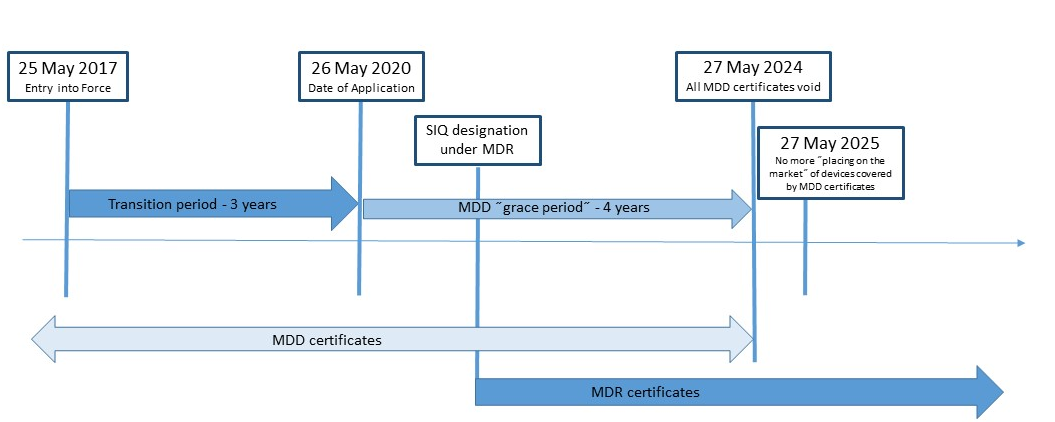
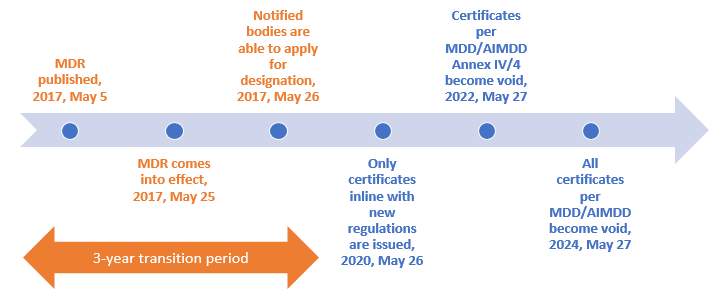
MDR 2017/745 - Article 120: Provisions on the marketing of devices and validity of EC certificates - Ente Certificazione Macchine

Safety reporting in clinical investigations of medical devices under the Regulation (EU) 2017/745 | SKYbrary Aviation Safety