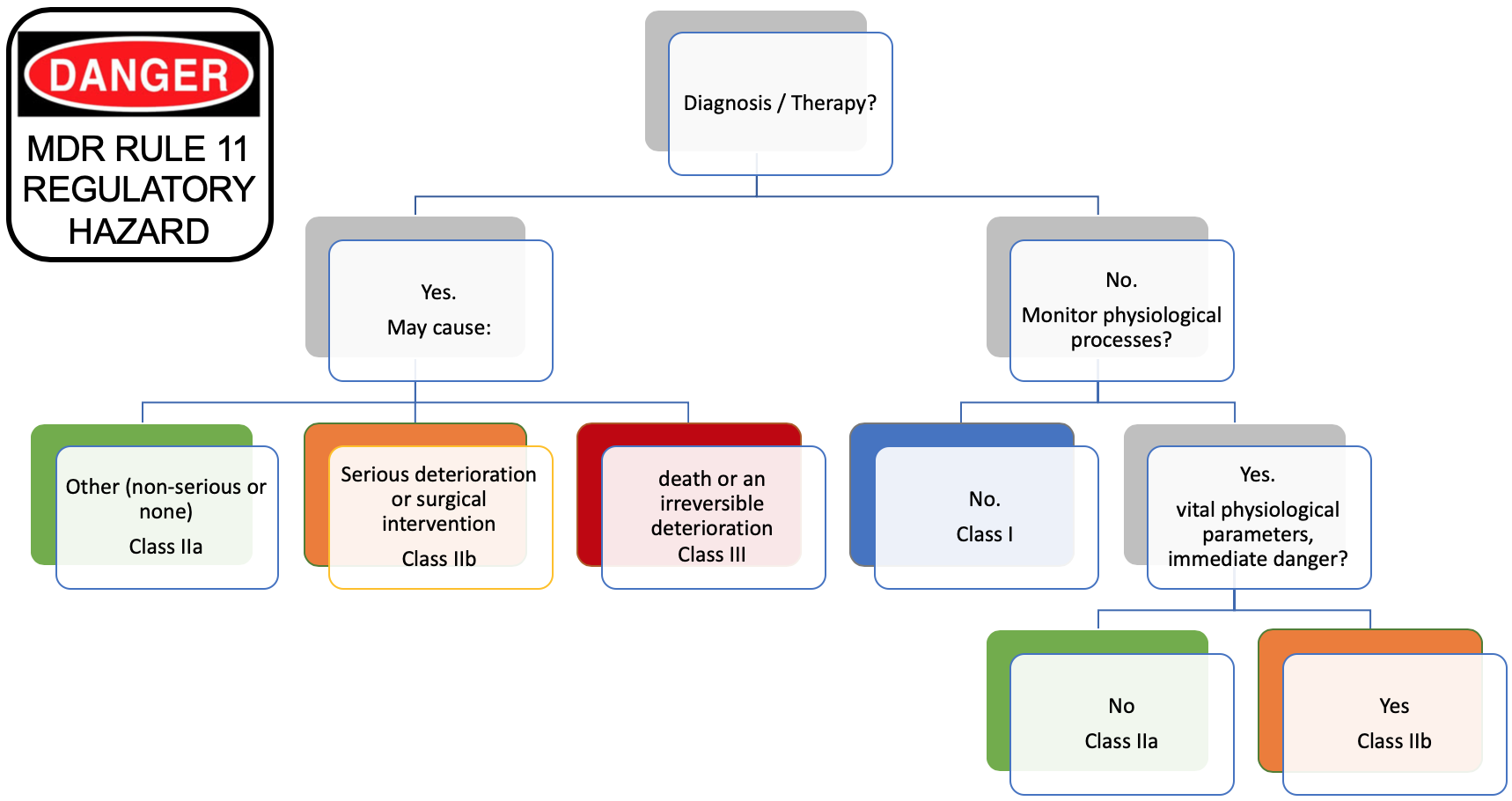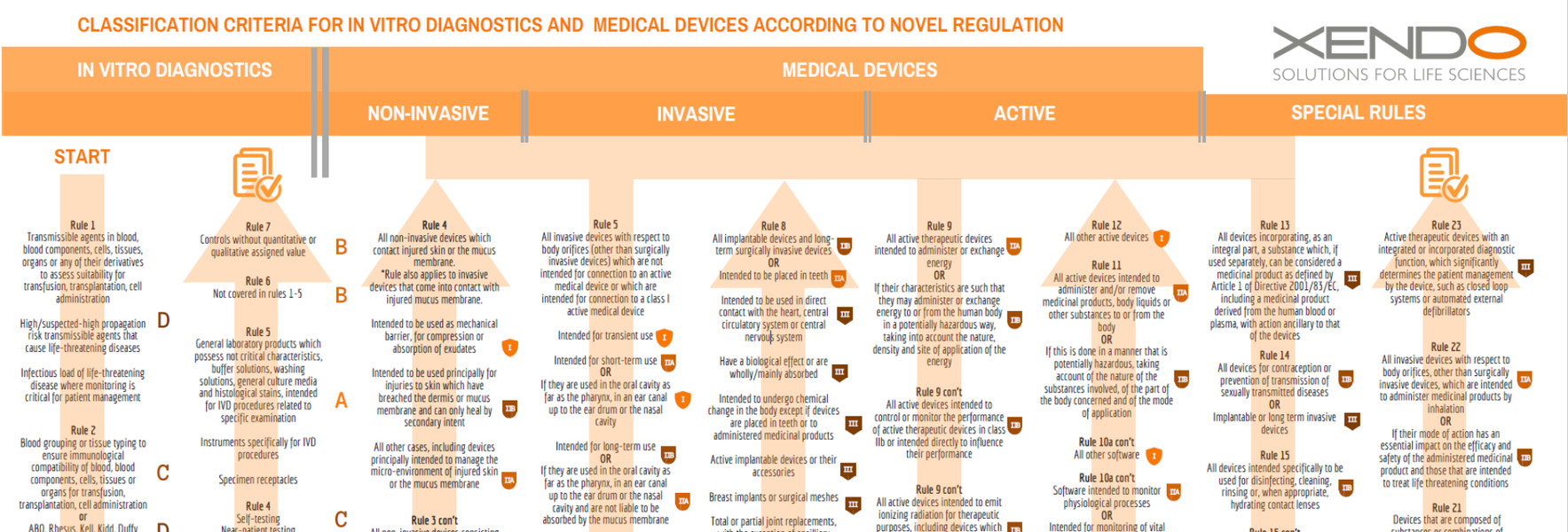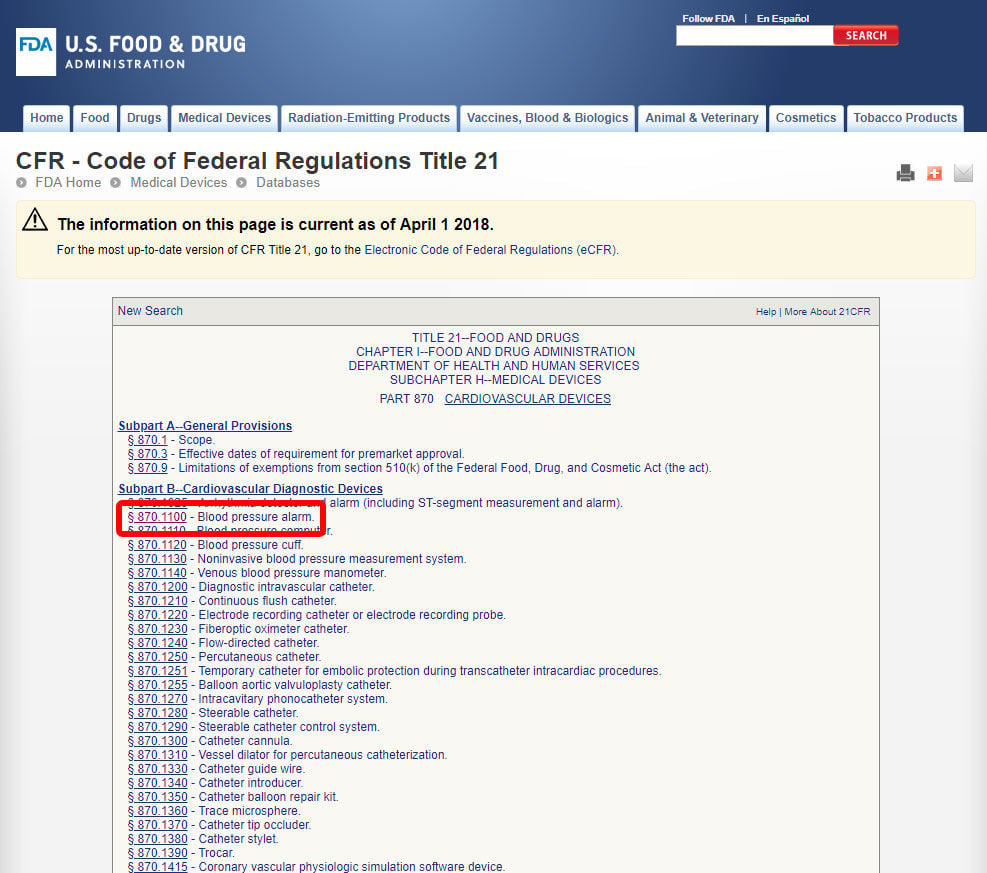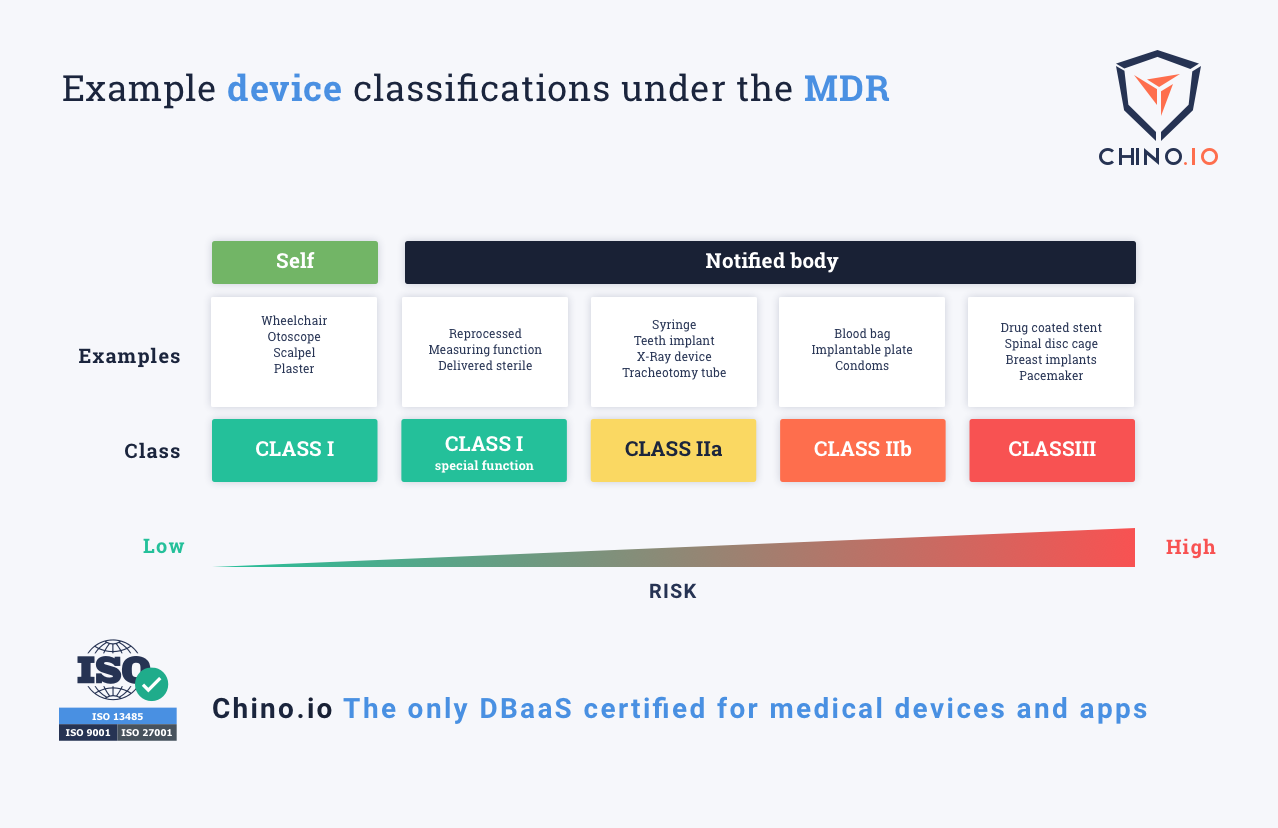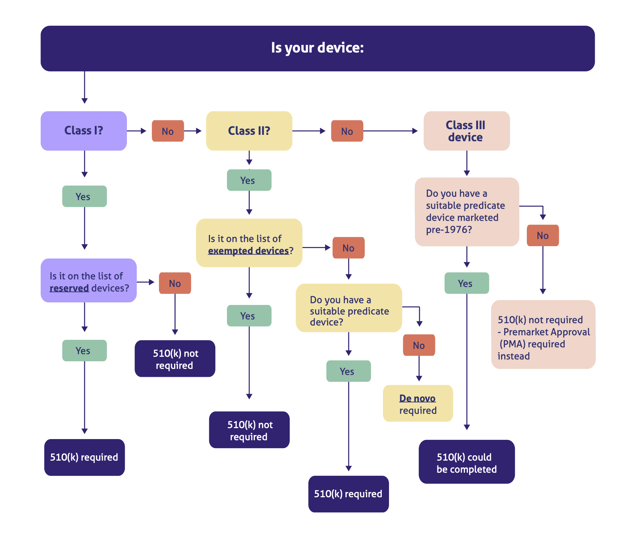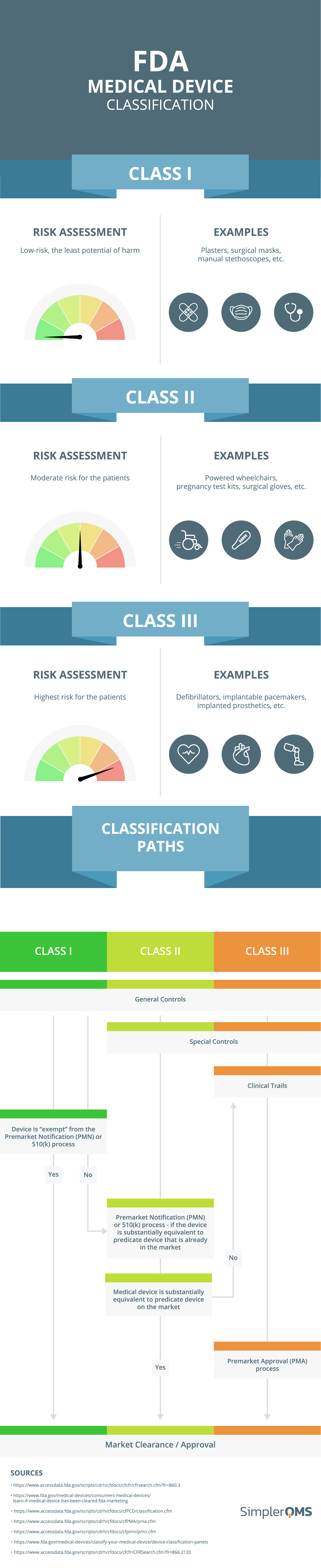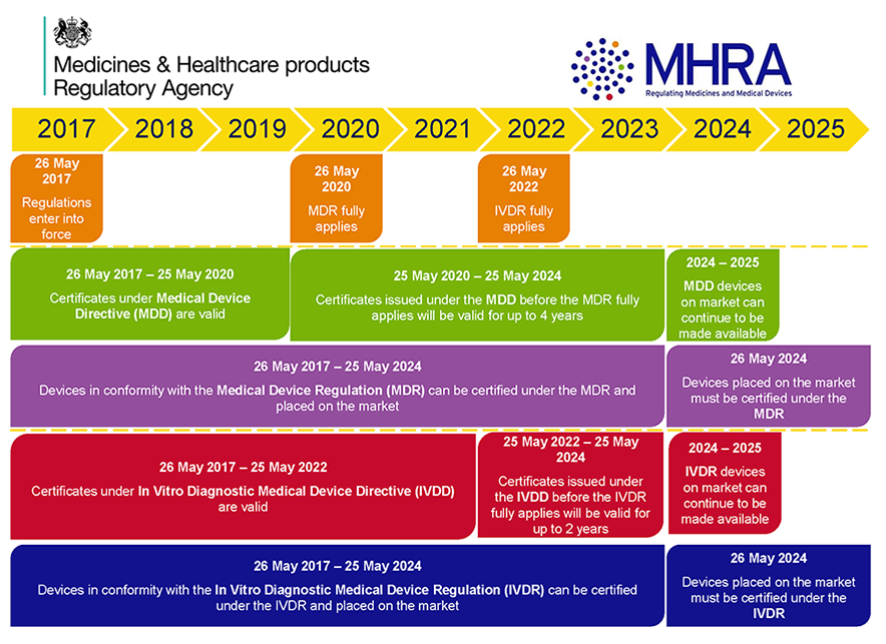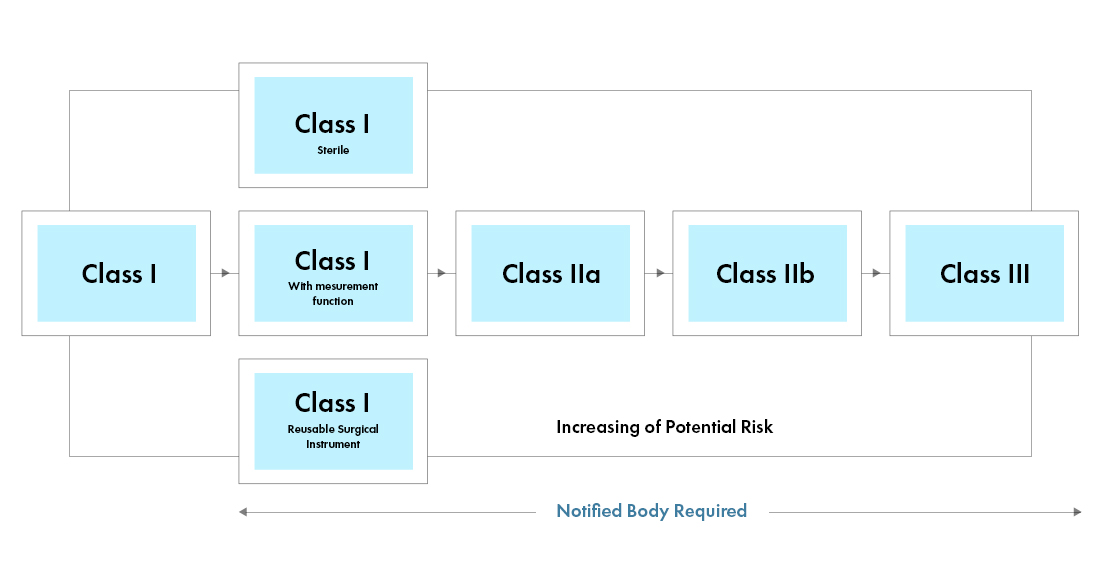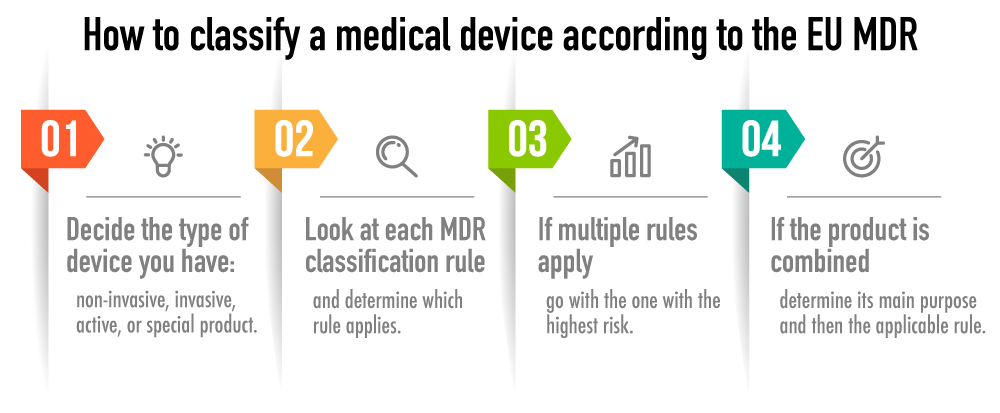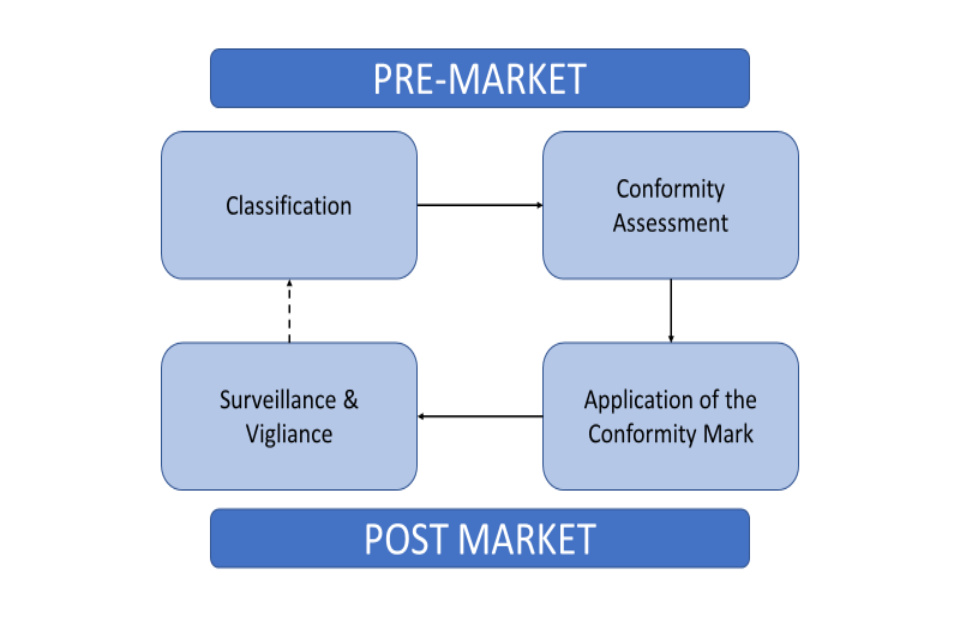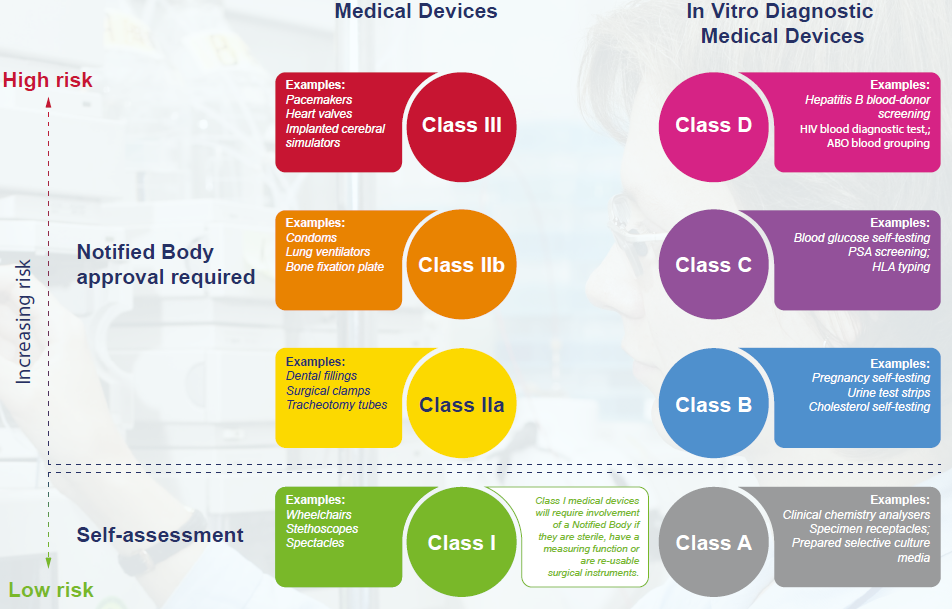
Interoperability standards for medical device integration in the OR and issues relating to international approval procedures (part 4) - ISCASBlog

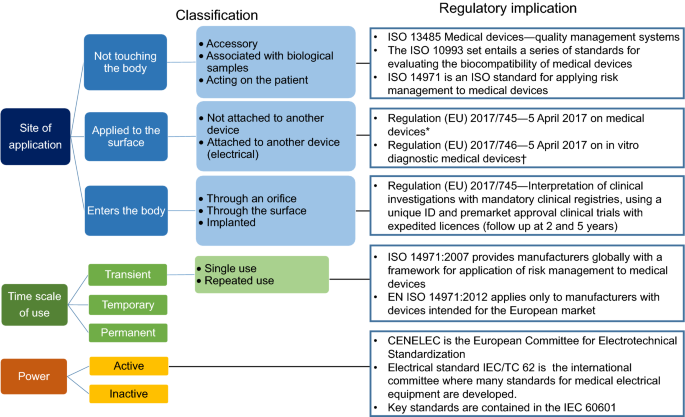
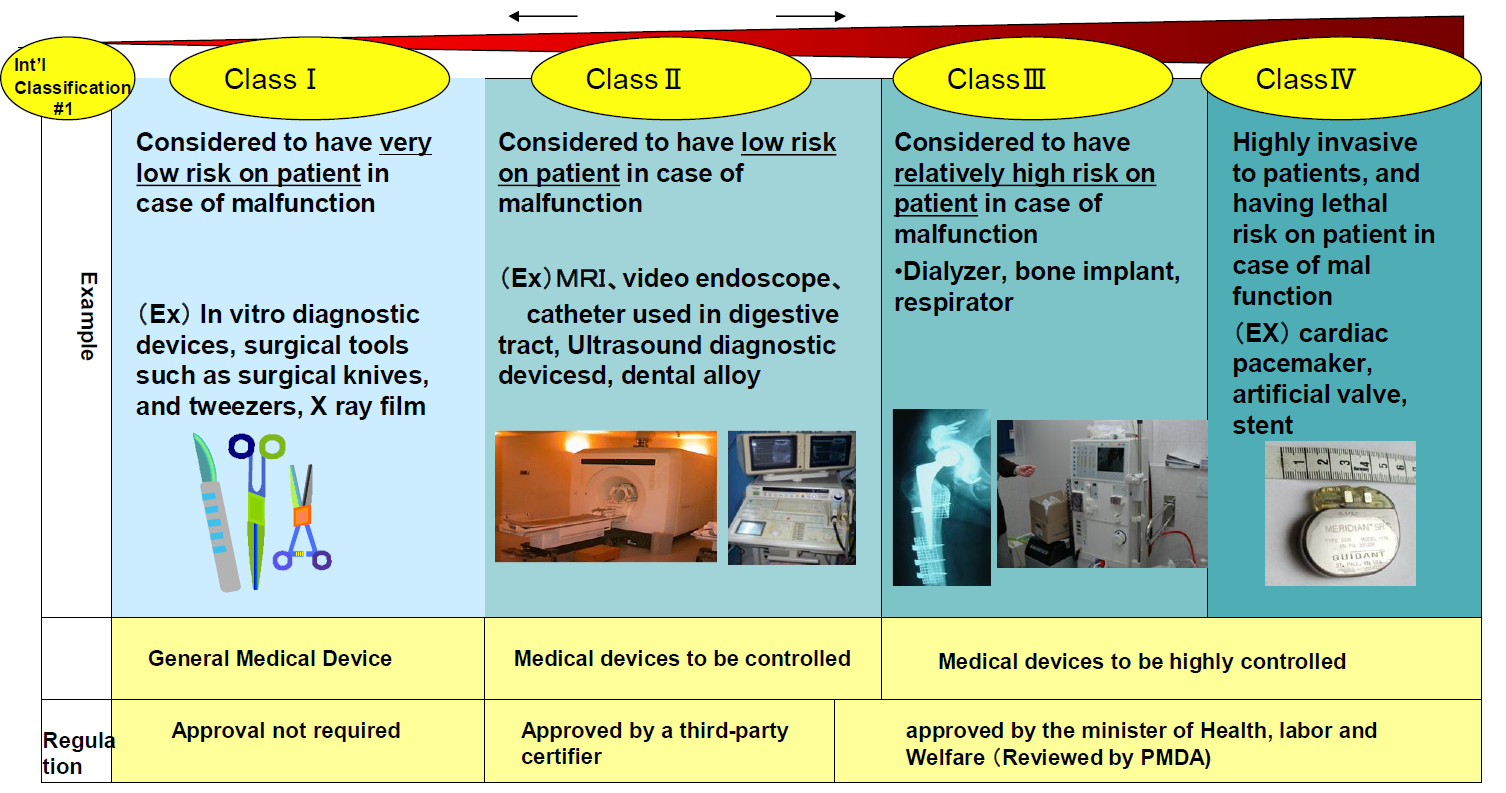
General classification and application types of medical devices for... | Download Scientific Diagram