In vitro diagnostic software: Novelties introduced by Regulation (EU) 2017/746 - GMED Medical Device Certification

In vitro Diagnostic Medical Device Regulation Archives · MDlaw – Information platform on European medical device regulations

Medical device regulation in Europe – what is changing and how can I become more involved? - EuroIntervention




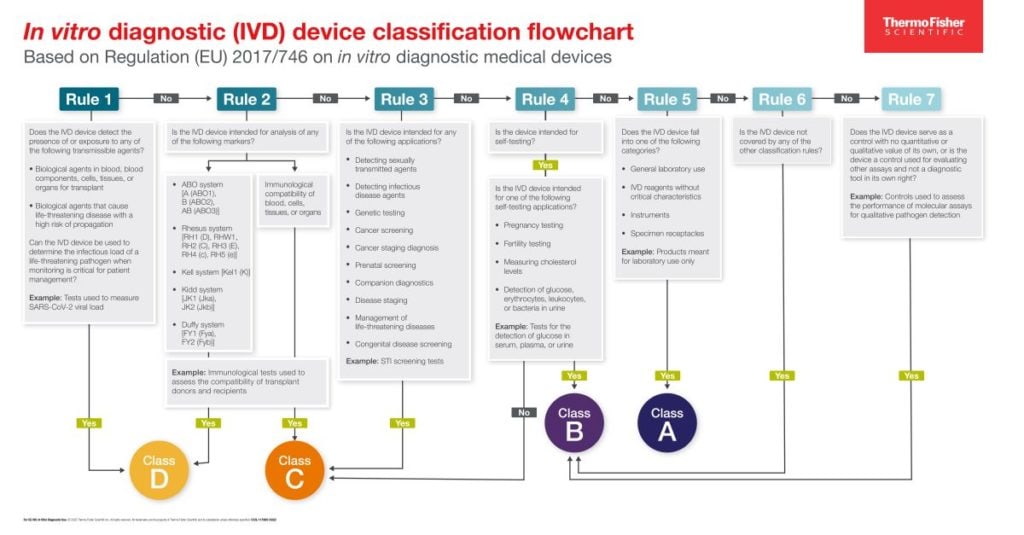



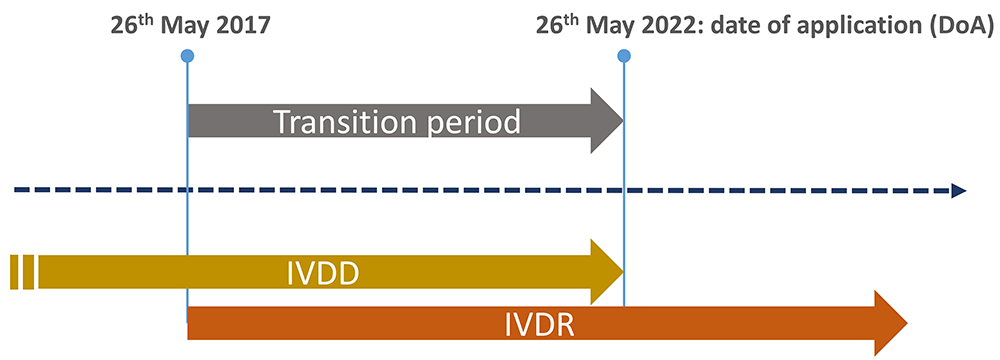
/tuv-rheinland-ivdr-visual-1-en_core_1_x.png)
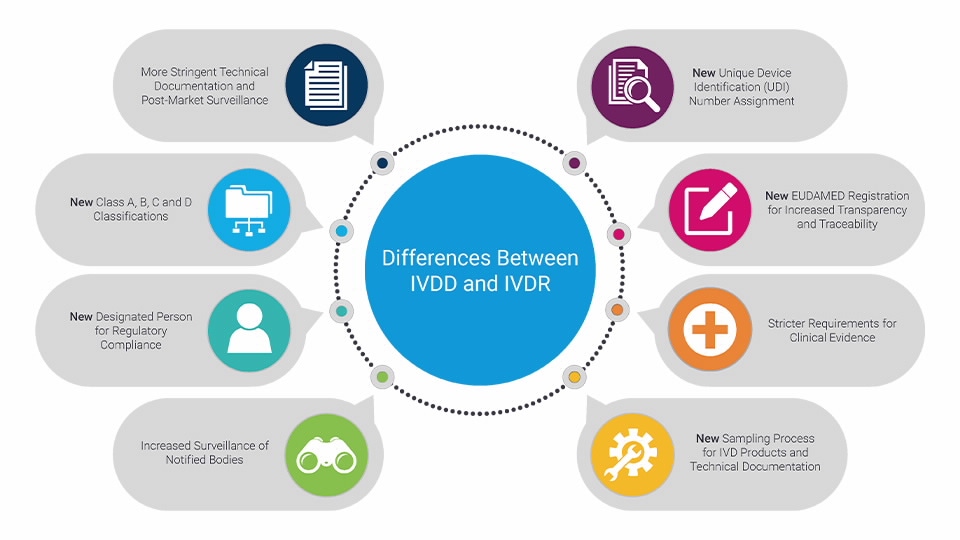





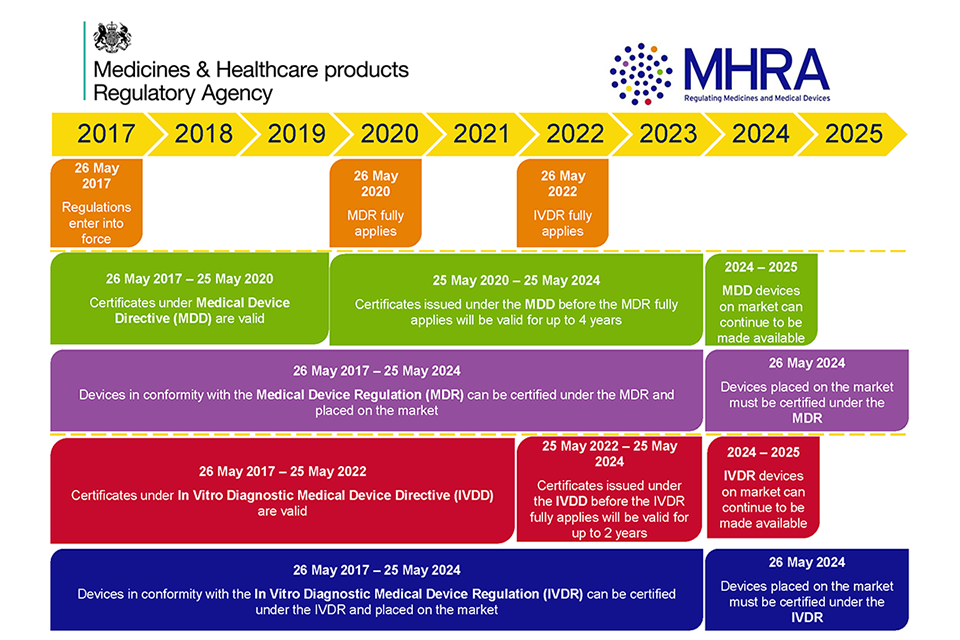
/tuv-rheinland-ivdr-visual-2-en_core_1_x.png)