Medical device regulation in Europe – what is changing and how can I become more involved? - EuroIntervention
![2021 MFDS-ACRS Conference] Drug Device Combination Products in the EU &The Impact of the New EU MDR - YouTube 2021 MFDS-ACRS Conference] Drug Device Combination Products in the EU &The Impact of the New EU MDR - YouTube](https://i.ytimg.com/vi/1-rRZ_0MkiA/maxresdefault.jpg)
2021 MFDS-ACRS Conference] Drug Device Combination Products in the EU &The Impact of the New EU MDR - YouTube
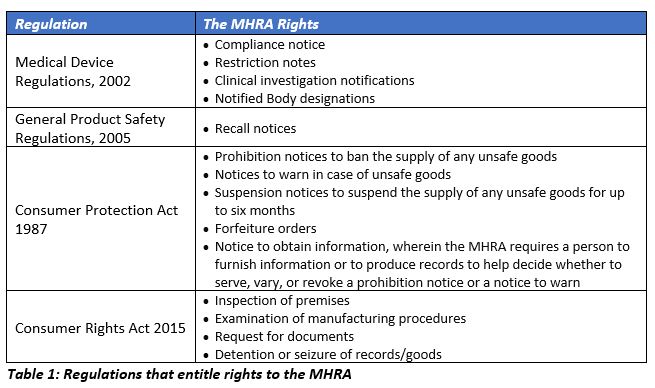
BREXIT – La @MHRAdevices actualiza los requisitos UK para los productos sanitarios a partir de 1 de Enero de 2021